When you write a prescription, do you reach for the brand name or the generic? It’s not just a habit-it’s a clinical decision with real financial, safety, and outcomes implications. For most medications, the answer should be clear: generic prescribing is the standard. But it’s not as simple as swapping a brand for a cheaper version. There are rules, exceptions, and patient concerns that every provider needs to understand to prescribe safely and effectively.
What Generic Prescribing Actually Means
Generic prescribing means writing the prescription using the International Non-proprietary Name (INN)-the official name of the active ingredient-instead of the brand name. So instead of writing "Lipitor," you write "atorvastatin." Instead of "Losec," you write "omeprazole." This isn’t new. The World Health Organization started the INN program in 1950 to standardize drug names worldwide. Since then, nearly every country has adopted it. In the UK, the NHS began pushing for generic prescribing in the 1990s to cut costs. Today, 89.7% of prescriptions in England are written generically, up from 86.2% in 2016. In the U.S., generics make up 90% of prescriptions dispensed, but only 20% of total drug spending. The reason? Cost. Generic drugs cost 80-85% less than their branded counterparts. Atorvastatin (generic) costs about £2.50 per month. Lipitor (brand) used to cost £30. Omeprazole (generic) is £1.80. Losec was £15. That’s not a small difference-it’s life-changing for patients on fixed incomes.Why Generics Are Just as Safe and Effective
A lot of patients worry that generics aren’t as good. They’re not. They’re the same drug. The FDA, EMA, and MHRA all require generics to meet strict standards:- Same active ingredient, strength, dosage form, and route of administration
- Same bioequivalence: 80-125% absorption rate compared to the brand
- Same manufacturing quality, purity, and potency
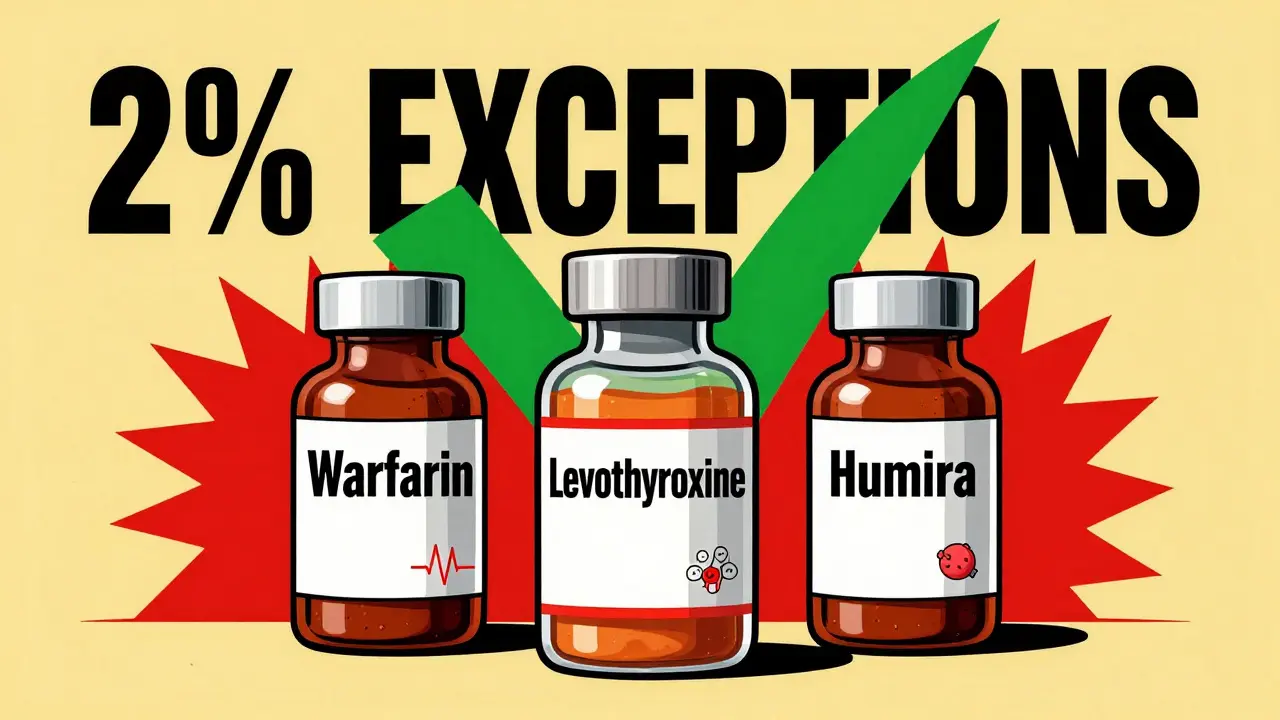
The Three Exceptions: When You Must Prescribe by Brand
This isn’t about being old-fashioned. There are real, evidence-based exceptions where switching brands can be risky. The British National Formulary (BNF) and MHRA clearly define three categories where brand-name prescribing is recommended:- Narrow therapeutic index drugs-where small changes in blood levels can cause harm or treatment failure. Examples: warfarin, levothyroxine, phenytoin, carbamazepine, digoxin. For warfarin, even a 10% shift in absorption can cause bleeding or clots. For levothyroxine, switching between generic manufacturers has been linked to INR and TSH fluctuations in sensitive patients.
- Modified-release formulations-drugs designed to release slowly over time. Examples: theophylline, some extended-release metformin or venlafaxine. The way the tablet breaks down can vary between manufacturers, affecting how the drug is absorbed. Pharmacists report 41% of them encounter issues with these.
- Biologics and biosimilars-complex proteins made from living cells. Examples: Humira, Enbrel, Remicade. Because they’re not chemically identical like small-molecule drugs, switching between originator and biosimilar can trigger immune reactions. The MHRA says: prescribe by brand name to avoid automatic substitution.
Why Patients Resist-And How to Handle It
You might have had this conversation: "But I’ve always taken the blue pill. This one looks different. Will it even work?" It’s not just ignorance. It’s the nocebo effect-the opposite of placebo. When patients believe a generic is inferior, they’re more likely to report side effects or feel it’s less effective. A 2021 study of 3,200 patients found that when doctors explained why generics were safe, acceptance jumped from 67% to 89%. Use this script: "This generic version has the exact same active ingredient as the brand you’ve been taking. It’s been tested to work the same way in your body. The only difference is the price-it’ll save you about £12 a month with no change in how well it works." Don’t say: "It’s cheaper." That invites skepticism. Say: "It’s the same drug, just without the brand name." Patients who’ve had bad experiences with generics often point to sertraline or levothyroxine. But studies show these are rare. In one NHS survey, only 9% of negative reviews mentioned levothyroxine, and 12% mentioned sertraline. Most of those patients switched manufacturers multiple times without monitoring. Consistency matters.How to Implement This in Practice
You don’t need to memorize 50 exceptions. Here’s how to make it easy:- Set your e-prescribing system to default to generics. Most systems let you lock in generic as the standard. Only override when necessary.
- Keep the BNF’s three categories handy. Print a quick-reference card or save it on your tablet. The NHS has a free toolkit with clickable lists of drugs that need brand prescribing.
- Don’t switch patients without telling them. If a patient is stable on a brand, don’t auto-switch them at the pharmacy unless they agree. Especially with antiepileptics. The American Epilepsy Society says unmonitored switches can increase breakthrough seizures by 2.1-fold.
- Monitor high-risk patients. For warfarin, check INR 1-2 weeks after switching. For levothyroxine, check TSH in 6-8 weeks. For modified-release drugs, watch for symptom changes.

What’s Changing in 2025
The rules are getting tighter. The MHRA updated its guidance in March 2023 to require brand-name prescribing for complex generics like glatiramer acetate, because manufacturing differences affect how the drug behaves. The FDA’s new GDUFA III rules require manufacturers to report any formulation changes that might impact absorption. That means more transparency. The NHS aims to get generic prescribing to 92% by March 2024. The focus now isn’t just on cost-it’s on smart substitution. Using real-world data to identify which patients can safely switch and which need brand continuity. That’s the future: personalized prescribing, not blanket rules.Bottom Line
Generic prescribing isn’t about cutting corners. It’s about using science to deliver better care at lower cost. For 98% of drugs, it’s safe, effective, and financially responsible. For the other 2%, the exceptions are clear, well-documented, and based on real patient risk. Start by setting your system to default to generics. Learn the three exceptions. Talk to your patients. Monitor those who need it. And remember: the goal isn’t just to save money-it’s to help more people stay on their meds, stay healthy, and avoid hospital visits.Are generic drugs really as effective as brand-name drugs?
Yes. Generic drugs must meet the same strict standards as brand-name drugs for active ingredients, strength, dosage form, and bioequivalence. Regulatory agencies like the FDA, EMA, and MHRA require generics to prove they work the same way in the body. Studies show no difference in effectiveness for the vast majority of medications. The only difference is cost-generics typically cost 80-85% less.
When should I avoid prescribing generics?
Avoid generic substitution for three types of drugs: 1) Narrow therapeutic index drugs like warfarin, levothyroxine, phenytoin, and carbamazepine-small changes in blood levels can be dangerous; 2) Modified-release formulations like extended-release theophylline or metformin, where absorption differences can affect dosing; and 3) Biologics like Humira or Enbrel, where switching between originator and biosimilar may trigger immune reactions. Always prescribe by brand name for these.
Why do some patients say generics don’t work for them?
Often, it’s the nocebo effect-patients believe generics are inferior, so they experience side effects or feel less effective, even when there’s no clinical difference. Studies show that when doctors explain that generics contain the same active ingredient and are tested to work the same way, patient acceptance increases from 67% to 89%. Rarely, switching between generic manufacturers for drugs like levothyroxine or sertraline can cause issues, especially if done repeatedly without monitoring.
Can I switch a patient from a brand to a generic without telling them?
No. Even if it’s clinically safe, you should always inform the patient. Many patients are attached to the brand they’ve been using, and surprise switches can cause anxiety or non-adherence. Always explain the switch, use the same script: "This is the same medicine, just without the brand name. It’s cheaper and works the same way." For high-risk drugs like antiepileptics or warfarin, never switch without a plan for monitoring.
How do I know which drugs have generic equivalents?
In the UK, the British National Formulary (BNF) lists all drugs and flags those with generic equivalents. In the U.S., the FDA’s Orange Book shows which drugs have approved generic versions and their therapeutic equivalence ratings. Most electronic prescribing systems automatically suggest generics and flag exceptions. You don’t need to memorize the list-just use your system’s defaults and review the three exception categories regularly.

pascal pantel
December 21, 2025 AT 07:17Let’s cut through the noise: if you’re still prescribing brand-name statins without a clinical justification, you’re either billing for waste or you’ve never opened a formulary. The FDA’s bioequivalence thresholds aren’t suggestions-they’re legally enforceable pharmacokinetic benchmarks. 80–125% AUC? That’s not ‘close enough’-that’s statistically indistinguishable from the originator in 99.7% of cases. And before you say ‘but my patient felt different,’ cite the JAMA study on adherence or shut up. This isn’t astrology, it’s pharmacology.
Gloria Parraz
December 22, 2025 AT 05:01I’ve had patients cry because they couldn’t afford their meds. One woman with hypertension skipped doses for three months because Lipitor cost her $48 a refill. When we switched her to atorvastatin? She showed up two weeks later with her BP down to 128/82 and a thank-you card. This isn’t about savings-it’s about dignity. Prescribing generics isn’t cutting corners. It’s giving people a chance to live.
Sahil jassy
December 22, 2025 AT 08:19Kathryn Featherstone
December 24, 2025 AT 03:16I used to avoid generics because I thought patients would feel like they were getting second-rate care. But after using the script from the post-'same medicine, no brand name'-I’ve seen acceptance rates climb. One elderly patient told me, 'I didn’t know it was the same thing.' That broke my heart. We’re not just prescribing drugs-we’re rebuilding trust. Small conversations matter.
Nicole Rutherford
December 25, 2025 AT 03:03Oh please. You think generics are magically safe? I had a patient on sertraline switch generics three times in six months. She developed tremors, insomnia, and suicidal ideation. The pharmacy didn’t even tell her it changed. And now you want to push this as a blanket policy? Wake up. Not all generics are created equal. The FDA doesn’t monitor every batch. Some manufacturers cut corners. And if you’re too lazy to check, don’t blame the patient when they crash.
Marsha Jentzsch
December 25, 2025 AT 09:38Ugh. I can’t believe you’re still pretending this is about science. It’s about corporate greed. The same companies that make the brand names own the generics. They just repackage the same damn pill and charge 10% of the price. And now they’re pushing this as a 'public health win'? Please. The real issue is that drug companies are allowed to monopolize for 20 years, then suddenly it’s 'oh, now it’s cheap!'-and we’re supposed to be grateful? This isn’t equity. It’s exploitation dressed up in a white coat.
Carolyn Benson
December 26, 2025 AT 16:05There’s a deeper philosophical question here: if a drug is chemically identical, is it still the same entity in the patient’s lived experience? The body doesn’t just absorb molecules-it absorbs meaning. The color, the shape, the brand logo-they’re part of the therapeutic ritual. To dismiss that as 'nocebo' is to reduce healing to a chemical equation. Maybe the real failure isn’t in the pill-it’s in our refusal to acknowledge that healing is a narrative, not just a pharmacokinetic curve.
Connie Zehner
December 27, 2025 AT 19:46Carolyn, you’re overcomplicating it. The patient doesn’t care about your philosophy-they care if they can afford to take their meds. And if they’re stable on a brand, don’t switch them. But if they’re struggling? Generic is the ethical choice. Stop hiding behind 'meaning' when the data says 98% of the time it’s identical. I’ve seen patients die because they skipped doses. You want to talk about narrative? Try telling their family why you didn’t help them afford the pill that could’ve kept them alive.