When you’re living with Crohn’s disease or ulcerative colitis, the goal isn’t just to manage symptoms-it’s to get your life back. For many, conventional treatments like steroids or immunomodulators stop working. That’s when IBD biologics come in. These aren’t your everyday pills. They’re precision-targeted drugs designed to shut down specific parts of your immune system that are attacking your gut. And they’ve changed everything for millions.
What Are IBD Biologics?
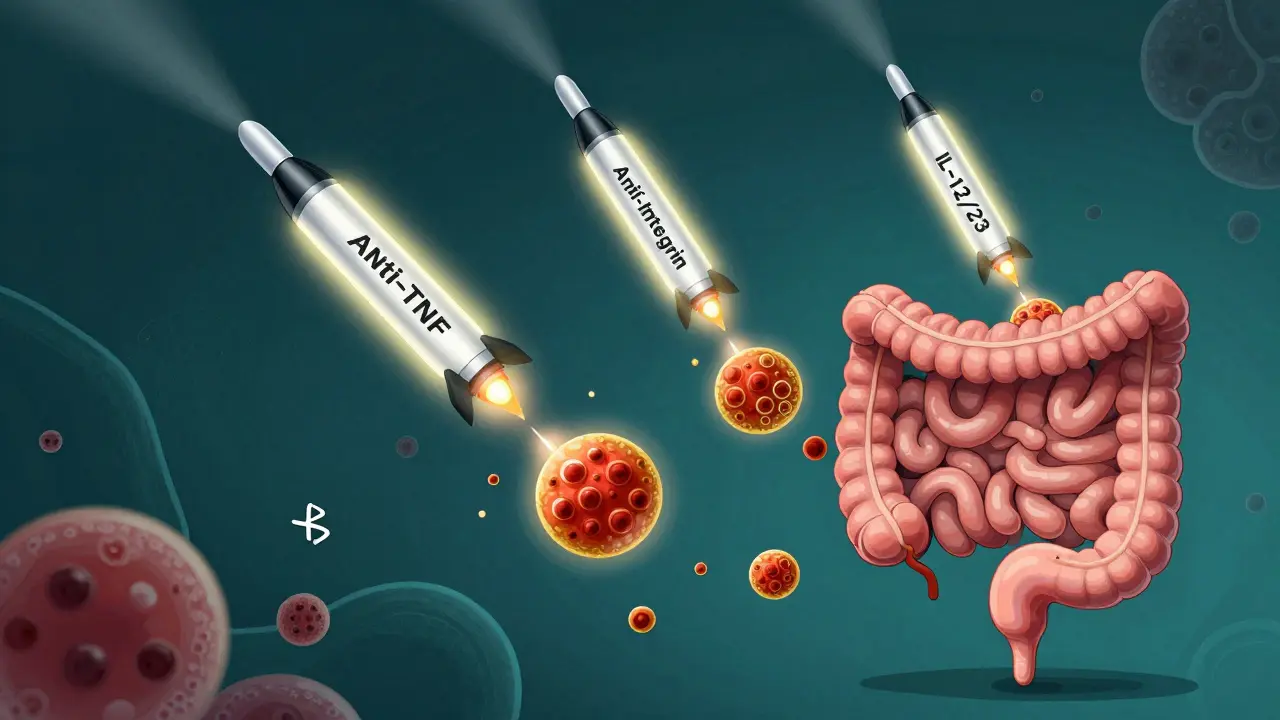
IBD biologics are made from living cells, not chemicals. They mimic proteins your body already makes to calm down an overactive immune response. Think of them as smart missiles: instead of blasting your whole immune system (like steroids do), they hit only the troublemakers-like TNF-alpha, integrins, or interleukins-that fuel inflammation in your intestines. They’re not new. Infliximab (Remicade) hit the market in 1998, the first real game-changer. Since then, we’ve seen three major classes emerge: anti-TNF agents, anti-integrins, and IL-12/23 inhibitors. Each works differently. Each has pros and cons. And choosing the right one isn’t just about science-it’s about your lifestyle, your risks, and your goals.Anti-TNF Agents: The First Line of Defense
Anti-TNF drugs were the pioneers. They block tumor necrosis factor-alpha, a key inflammatory signal in IBD. This class includes infliximab (Remicade), adalimumab (Humira), golimumab (Simponi), and certolizumab pegol (Cimzia). Infliximab is given as an IV infusion-about two to four hours every eight weeks after an initial three-dose kickstart. Adalimumab is a self-injection under the skin every other week. Both are proven to get people into remission, heal the gut lining, and reduce hospitalizations. But here’s the catch: they’re not equally effective for everyone. A 2022 meta-analysis showed infliximab had higher remission rates than adalimumab in patients who’d never tried a biologic before. For moderate to severe ulcerative colitis, infliximab was ranked best for inducing remission. But in real life, many patients choose adalimumab because they don’t want to spend half a day at a clinic every month. Side effects? Higher risk of serious infections like tuberculosis or pneumonia. Some people get infusion reactions-rashes, fever, chills. About 0.5% have severe allergic reactions. And over time, your body might make antibodies that stop the drug from working. That’s called loss of response. It happens in 6-25% of users. Biosimilars-cheaper copies of these drugs-are now widely available. Inflectra and Cyltezo offer the same effect at 15-30% less cost. Many insurance plans push them first.Anti-Integrin Therapies: Gut-Selective and Safer
Vedolizumab (Entyvio) is the only anti-integrin approved for IBD in the U.S. It works differently. Instead of suppressing your whole immune system, it stops white blood cells from entering your gut. It’s like putting up a roadblock just where the damage is happening. That makes it safer. No increased risk of brain infections like PML (a rare but deadly side effect of another drug, natalizumab). No higher cancer risk. No reactivation of hepatitis B. It’s the go-to for patients with a history of MS, TB, or those worried about systemic side effects. You get it as an IV infusion-same schedule as infliximab: weeks 0, 2, 6, then every 8 weeks. But it takes longer to work. Most patients don’t feel better until 6-10 weeks in. One Reddit user wrote, “I waited 10 weeks. Felt like I was dying the whole time.” Still, patient satisfaction is high. On MyIBDTeam, 72% said it worked for them, and only 18% reported side effects-far lower than anti-TNFs. The trade-off? Slower results. If you’re in severe pain and need fast relief, this might not be your first pick. But if you’re looking for long-term safety and steady control, it’s a top contender.IL-12/23 and IL-23 Inhibitors: The New Generation
Ustekinumab (Stelara) was approved for Crohn’s in 2016 and ulcerative colitis in 2019. It blocks IL-12 and IL-23, two cytokines involved in chronic inflammation. You get it as a subcutaneous injection-either every 8 or 12 weeks, depending on your weight. Then came the IL-23-only inhibitors: risankizumab (Skyrizi) and mirikizumab (Omvoh). Risankizumab got FDA approval for ulcerative colitis in June 2024, making it the first drug in its class approved for both Crohn’s and UC. Mirikizumab was approved for UC in 2022. These drugs are powerful. In clinical trials, risankizumab put 29% of UC patients into remission at one year-compared to just 10% on placebo. They’re also cleaner. Fewer infections. No black box warnings. No need for TB screening before starting. They’re self-injected monthly or every other month. No clinics. No IVs. That’s a huge win for people juggling work, kids, or long commutes. Cost? A single 130mg dose of ustekinumab runs about $7,200. A 300mg dose of vedolizumab is around $5,500. But most patients pay far less thanks to manufacturer assistance programs. Janssen, AbbVie, and Takeda all offer $0 copay programs for eligible patients.Which One Should You Choose?
There’s no one-size-fits-all. But here’s how top experts think about it:- If you have severe disease and need fast results? Infliximab still leads in the data.
- If you hate clinics and want control at home? Adalimumab or ustekinumab win on convenience.
- If you have psoriasis, a history of TB, or are nervous about systemic risks? Vedolizumab is the safest bet.
- If you’ve tried other biologics and failed? Risankizumab or mirikizumab offer new hope.
Real Talk: What Patients Say
On forums like Reddit and MyIBDTeam, the stories are raw:- “Remicade worked in two weeks-but the 8-hour round trip every month? I couldn’t keep doing it.”
- “I switched from Humira to Entyvio after five years. No more injection site burns. But I cried for weeks waiting for it to kick in.”
- “Skyrizi changed my life. No more hospital visits. No more fear of infections. I’m finally sleeping through the night.”

What You Need to Know Before Starting
Before you begin any biologic:- Get all vaccines up to date. No live vaccines (like MMR or shingles) after you start.
- Get tested for TB and hepatitis B.
- Learn how to inject if you’re going self-administered. Most clinics offer training. Don’t skip it.
- Know the signs of infection: fever, chills, cough, unusual fatigue. Call your doctor immediately.
- Track your symptoms. Apps like MyTherapy help with reminders and logs. 68% of users say it improves adherence.

Alex LaVey
February 5, 2026 AT 08:28Man, this post nailed it. I’ve been on Humira for 4 years, switched to Stelara after my body stopped responding, and honestly? My life changed. No more ER trips. No more missing work. I’m hiking again, cooking for my kids, sleeping through the night. It’s not perfect-still got the occasional stomach bug-but compared to before? Night and day.
I know some folks worry about side effects, but the real trade-off is between suffering silently and taking a shot that lets you live. I’d rather risk a cold than spend another week curled up on the bathroom floor.
Also, shoutout to biosimilars. My insurance pushed me to Inflectra last year. Same drug, half the price. No difference in how I feel. Why pay more when it’s the same medicine?
Justin Fauth
February 5, 2026 AT 13:59Ugh. Another pharma love letter. You act like these drugs are miracles when they’re just expensive bandaids with black box warnings. I’ve seen three guys in my support group die from infections after starting biologics. One had PML. Another got sepsis from a simple UTI. They didn’t even get remission. Just died faster with a fancy label.
And don’t get me started on the ‘patient assistance programs.’ They’re a trap. You qualify one year, next year they change the rules, and suddenly you’re paying $8,000 a month. Big Pharma doesn’t care if you live or die-they care about stock prices.
Stop selling this like it’s salvation. It’s a gamble with your life.
Meenal Khurana
February 6, 2026 AT 05:58Thank you for this. Clear and helpful.
Zachary French
February 6, 2026 AT 08:31Okay so like… I read this whole thing and I’m just sitting here thinking-WHY ISN’T EVERYONE ON SKYRIZI?!?!? Like, come on. No infusions. No TB screenings. No hospital visits. 29% remission rate? That’s like, 3x better than placebo. Who’s still clinging to Remicade like it’s 2005? I swear, if my GI still pushed me to do IVs, I’d ghost them.
And also-MIRIKIZUMAB IS COMING? LIKE, WHEN? I NEED IT YESTERDAY. I’M SICK OF BEING A HUMAN POUCH FOR INFLIXIMAB. I’M A 32-YEAR-OLD WITH A JOB AND A DOG, NOT A CLINIC’S FAVORITE PATIENT.
Also, biosimilars are fire. Why are people still paying full price? Are you rich? Are you dumb? Are you both? I don’t get it.
Demetria Morris
February 7, 2026 AT 19:09It’s irresponsible to present biologics as a simple solution without emphasizing the moral weight of choosing them. You’re not just treating inflammation-you’re altering your body’s natural defenses. And for what? A few extra months of comfort? I’ve seen people sacrifice their immune systems for convenience, then wonder why they got shingles at 28.
There’s a reason these drugs come with black box warnings. It’s not a footnote. It’s a red flag. And yet, we treat them like coffee shop upgrades. ‘Oh, I’m just going to try Stelara.’ No. It’s not a flavor.
Before you start, ask yourself: Am I choosing life-or just avoiding discomfort? Because those are two very different things.
Caleb Sutton
February 8, 2026 AT 01:27They’re tracking you. Every injection. Every infusion. Every blood test. They’re building a profile. The CDC, the FDA, the insurance companies-they all know who’s on what biologic. Why? So they can deny you coverage later. Or worse-deny you a job. Or a visa. Or life insurance.
And don’t tell me it’s paranoia. The VA stopped giving biologics to veterans after 2021 because they were ‘too expensive to monitor.’ Coincidence? I think not.
They want you dependent. They want you docile. And they want your data.
Susheel Sharma
February 9, 2026 AT 04:41Interesting breakdown, but you missed a critical point: cost isn’t just about out-of-pocket-it’s about opportunity cost. Time off work for infusions. Transportation. Childcare. Lost wages. A 4-hour IV session costs $500 in lost income alone. For minimum wage workers? That’s two days’ pay. No one talks about this.
And the ‘$0 copay’ programs? They’re only for those with commercial insurance. Medicaid? Medicare? No luck. So you’re telling me a single mom on Medicaid in Texas has to choose between food and her medication? That’s not healthcare. That’s cruelty dressed up as innovation.
Jhoantan Moreira
February 9, 2026 AT 20:53Thank you for this. Really. As someone who’s been on vedolizumab for 3 years, I can say it’s been the quiet hero of my journey. Slow? Yes. Worth it? Absolutely.
I used to dread every clinic visit. Now, I go in, sit quietly, read a book, and leave. No burning, no fever, no scary lab results. I’m not ‘cured’-but I’m alive. And that’s enough.
To anyone scared of starting: it’s okay to take your time. You don’t have to rush into something because the internet says it’s ‘the best.’ Find what fits your rhythm. Your body, your life, your pace.
❤️
Daz Leonheart
February 10, 2026 AT 05:21Hey, just wanted to say-this post is gold. I’ve been reading everything I can on biologics since my diagnosis last year, and this is hands-down the clearest thing I’ve found. No fluff. No jargon. Just facts with heart.
I’m on adalimumab now. Took me 3 months to get used to the injections. Still hate them. But I’ve gone from 6 bathroom trips a day to 1-2. I’m back at work. I hugged my niece without worrying about pain.
You’re not alone. And you’re not broken. You’re just healing.
Amit Jain
February 11, 2026 AT 17:39Simple: if you need fast results, go infliximab. If you want safety, go vedolizumab. If you want easy, go ustekinumab or skyrizi. No magic. Just science. Pick what fits your life.
Mandy Vodak-Marotta
February 12, 2026 AT 13:59Okay, so I’ve been on this journey for 7 years-Crohn’s since I was 19. Started with steroids. Then azathioprine. Then Humira. Then Remicade. Then Entyvio. Now I’m on Skyrizi. And I’m not even mad anymore. Honestly? I’m kind of proud.
People think I’m ‘giving up’ by trying all these drugs. Nah. I’m trying everything so I don’t die. And I’m still here. Still working. Still traveling. Still laughing at dumb TikToks.
And yeah, the cost is insane. I’ve cried over bills. I’ve skipped doses. I’ve begged for help. But I never stopped fighting. And if you’re reading this and you’re scared? I get it. I was too.
But here’s the thing: you’re not just fighting your gut. You’re fighting for your future self. And that future self? She’s gonna thank you for not giving up.
Also, if you need someone to vent to? DM me. I’ve got 7 years of trauma, memes, and free samples. I’ve got room.